Topic outline
-
Here you will learn about radioactive isotopes, how they are used in medicine and learn how to make them using column chromatography.
-
Please print out a copy of the student handbook.
Fill in the blanks and answer the questions as you work through the e-learning course and in-class material.
-
You should already know about isotopes, but what are radioactive isotopes?
Watch the video below to find out more.
-
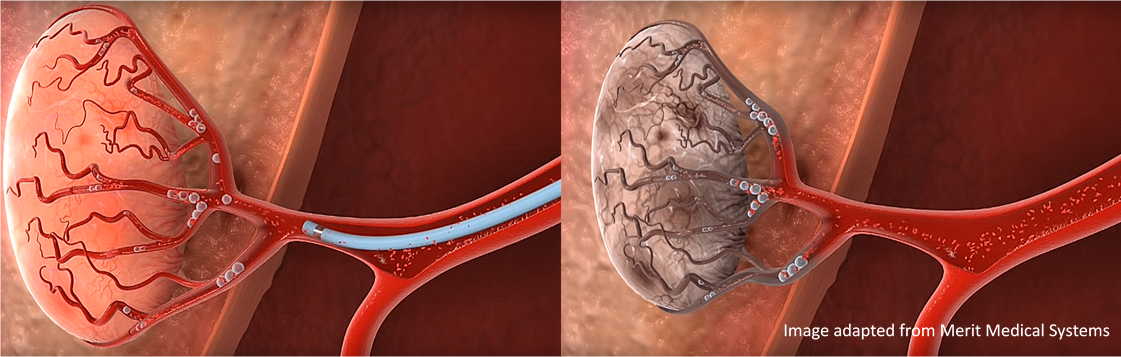
Yttrium-90 (90Y) is an important radioisotope that can be used to treat liver cancer. A catheter is used to insert tiny glass beads or microspheres filled with 90Y into the liver’s blood stream. These spheres are carried to the cancer where they become lodged in the small blood vessels surrounding it. The spheres then deliver a high dose of radiation, whilst starving the cancer of its blood supply – thereby killing it. This targeted delivery of radiation kills the cancerous cells while minimising exposure to healthy tissue.
90Y is formed when strontium-90 (90Sr) undergoes radioactive beta minus decay (β-). 90Y also undergoes β- decay to form stable zirconium-90.
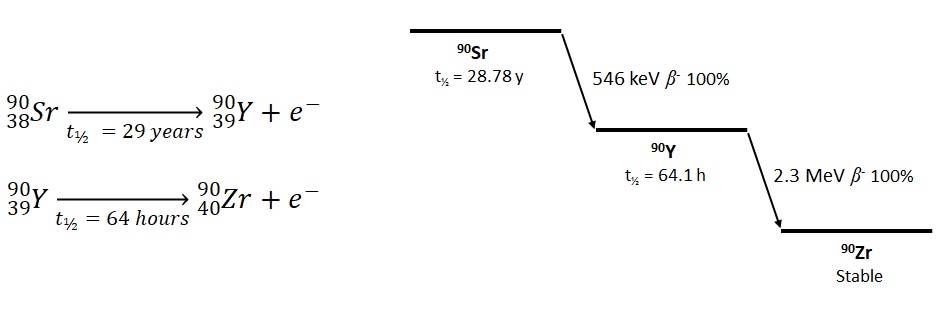
The short half-life (t½) of 90Y means that it can be implanted in the cancer and deliver a high dose of radiation in a relatively short time period. 90Sr has a longer half-life and would require a lot more material to deliver a similar radiation dose. Additionally Sr and Y behave differently in the body when washed out of the glass beads. Y is filtered by the kidneys and passed out as urine through the bladder over a few days. On the other hand, Sr can accumulate in bones where it would remain until it had decayed (~29 years), delivering a steady radiation dose. This can eventually lead to bone cancer and cancer of the surrounding tissues.
It is therefore very important to be able to separate these two isotopes. In your next chemistry class you will carry out an experiment to separate 90Sr and 90Y using column chromatography. Below, you will learn the theory behind this experiment to help you in class.
-
Chromatography provides an important method of separating, purifying and identifying components in a mixture.
Types of chromatography include:
- Thin-Layer Chromatography (TLC)
- Column Chromatography (CC)
- Gas Chromatography (GC)
All forms of chromatography work on a similar principle. They all have a stationary phase (a solid, or a liquid supported on a solid) and a mobile phase (a liquid or a gas). The mobile phase flows through the stationary phase and carries the components of the mixture with it. Each substance travels at a different rate depending on its solubility in the mobile phase and its retention (retardation) by the stationary phase.
-
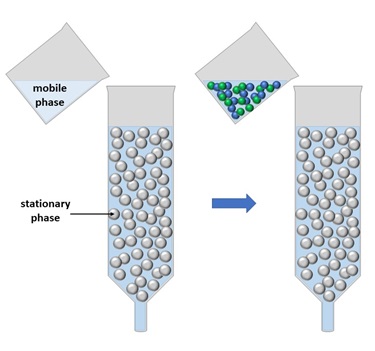
In column chromatography, a glass column is packed with an absorbent material (e.g. silica, ion exchange resin). This is the stationary phase.
A solvent (water, acid or organic (oil)) is then added to cover the stationary phase and ‘wet’ the entire column – this is how you set up the experiment.
The mixture to be separated (e.g. radioactive solution) is then carefully added to the top of the column.
The solvent is then allowed to run through the column, slowly and continually. The solvent is the mobile phase. It is important that the column never becomes ‘dry’ otherwise it can affect the separation.
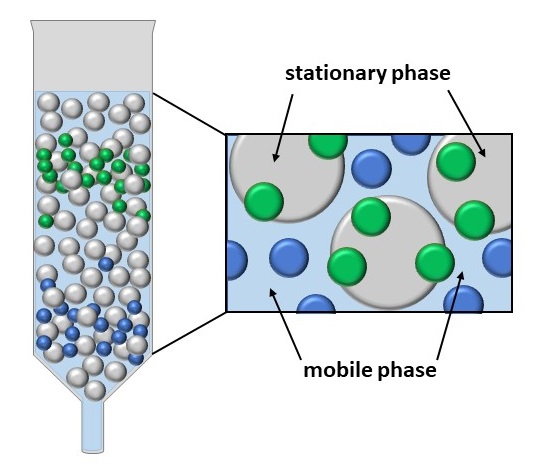
As the mobile phase runs though the column, the components of the mixture separate out as they move at different rates. This rate depends on how soluble each component is in the mobile phase and how strongly it is retained (adsorbed) by the stationary phase.
Components that adsorb strongly to the stationary phase will take a long time to travel down the column. Components that are very soluble in the mobile phase will pass through the column quickly. So it is a trade-off between how much a component sticks to the surface versus how quickly it is washed through by the solvent.
Time taken for each component to reach the end of the column is known as the Retention Time.
-
In your next chemistry lesson, you will separate two radioisotopes (90Sr and 90Y) using a form of column chromatography. Radioactive materials can be dangerous when handled improperly so you will use a robot to remotely handle the radioactive material. The experiment is actually set up in a laboratory in Germany!
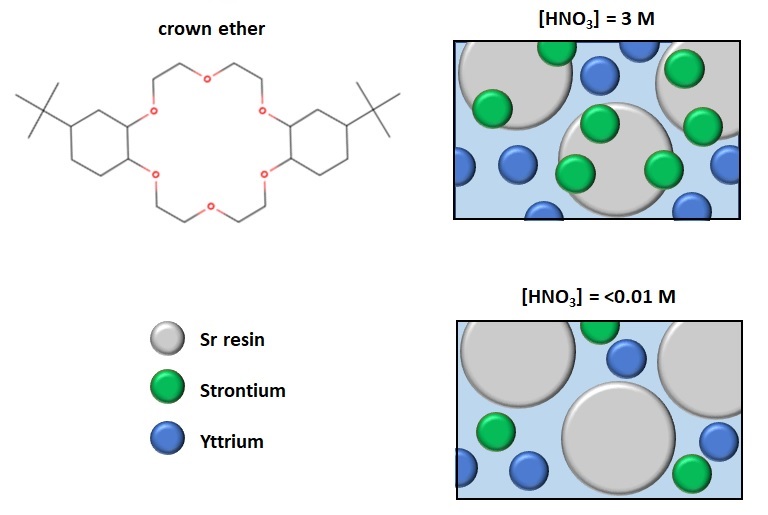
You will inject a radioactive solution of 90Sr and 90Y onto a separation column. The column is loaded with a crown-ether based ion exchange resin (called Sr resin). At high nitric acid (HNO3) concentrations (>2 M ) strontium adsorbs strongly onto the resin. However, at low concentrations (<0.01 M) it will readily pass through the column. This is due to the resin’s retention factor (k’):
-
The retention factor (k' ) describes the migration rate of an analyte on a column. k’ is a measure of the time the analyte component (90Y or 90Sr) resides in the stationary phase relative to the time it resides in the mobile phase. If the analyte resides mainly in the stationary phase, then k’ is large. If the analyte resides in the mobile phase and moves quickly through the ion exchange resin, k’ is small.
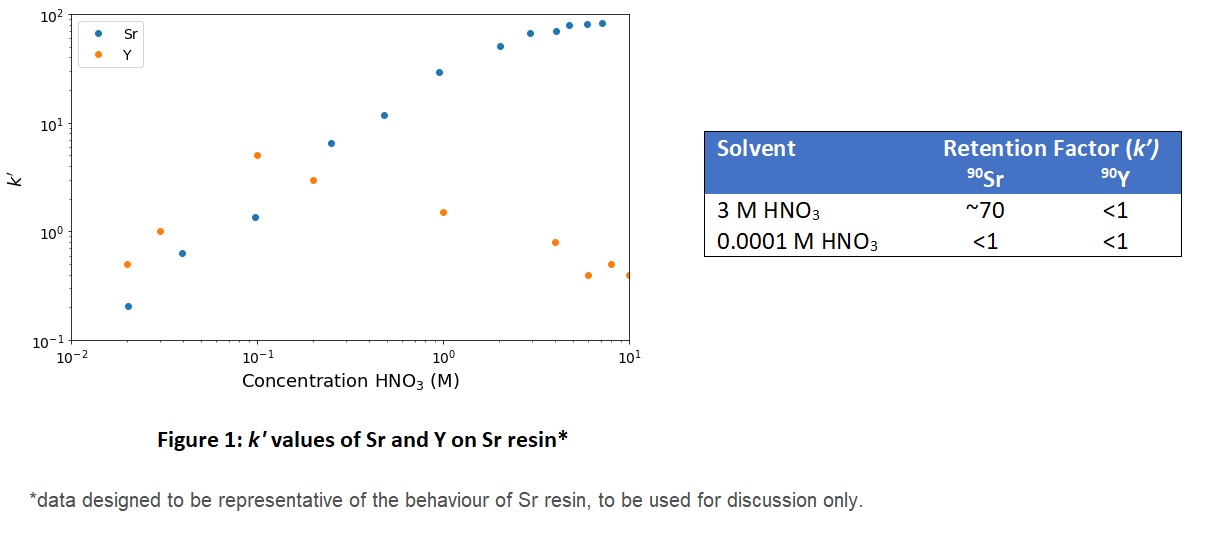
In this experiment, the concentration of nitric acid (HNO3) affects the rate at which 90Sr passes through the column. In figure 1 below, you can see that at high nitric acid concentrations, k' is large for Sr 2+ ; indicating that strontium has adsorbed onto the stationary phase. At low concentrations Sr moves into the mobile phase and can exit the column (k' is small).
-
In your next chemistry lesson you will be carrying out a column chromatography experiment, called "Ionlab".
Examine the experimental set-up below. Once you have familiarised yourself, use your understanding of the theory to complete the experimental procedure.
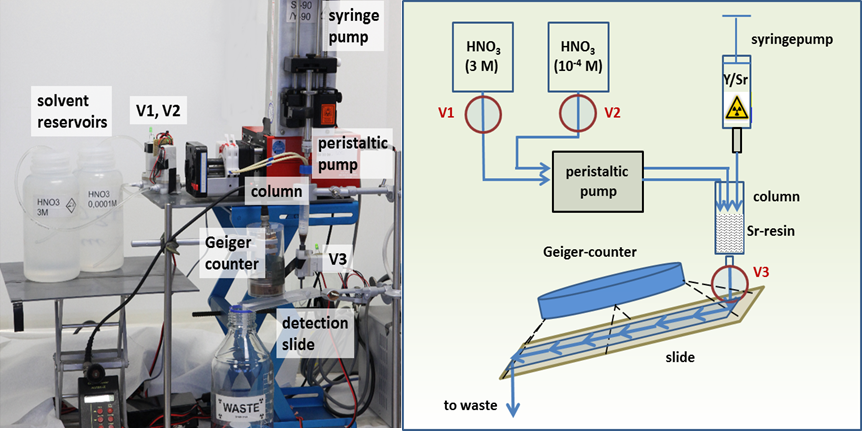
Apparatus
- Solution containing 90Sr and 90Y
- Syringe (to inject the solution)
- Three valves to control the solvent (V1, V2, V3)
- Peristaltic pump
- Solvent bottles (3 M and 0.0001 M HNO3)
- Separation Column (ion exchange resin)
- Geiger Counter (measures ionising radiation)
- Waste container
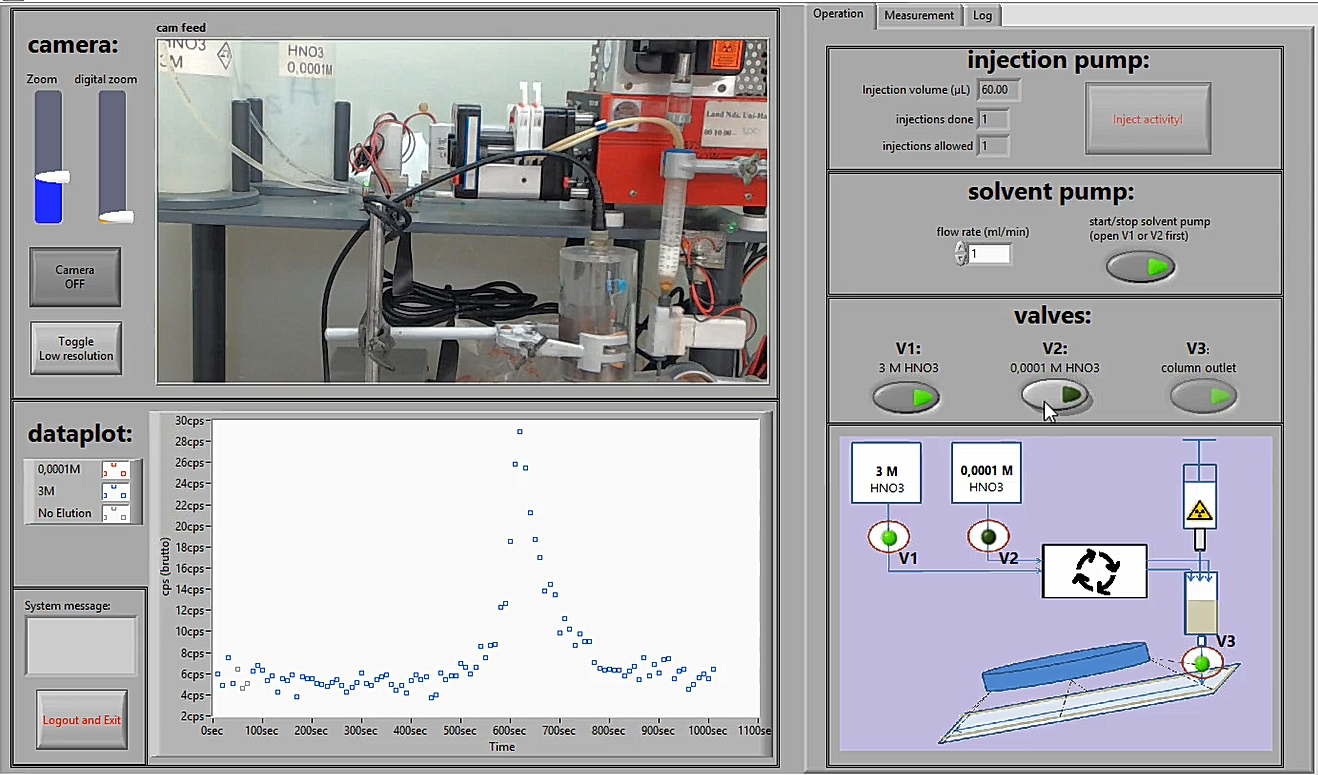
Look at the picture of the user interface below, this is how you will control the robot.
-
Use your understanding of column chromatography to complete the experimental procedure test below, or fill in the blanks in your hand notes.
Prepare the column
Open valve 3 (V3) in order to start the experiment.
Set the solvent flow rate to ________.
Select _________ solvent. (Hint: which solvent needs to run through the column first to separate 90Sr from 90Y?)
Click on “start solvent pump” to begin pumping the solvent onto the column. Use the camera feed to check that the pump is rotating and droplets of solvent are landing on the slide.
Allow the solvent to run for 5 minutes to __________.
Whilst waiting, set up your measurement parameters by going to the measurement tab. Set the measurement time to 3600 seconds (the duration of your experiment, 1 hour) and the counting interval to 10 seconds (this is how often the Geiger counter will record the activity).
Click on “start measurement”.
Make a note of the _________.
Separation of 90Y from 90Sr
9. After 5 minutes, stop the solvent pump.10. Click on ________________. The syringe will administer the 90Sr/90Y solution onto the top of the column.
11. Start the solvent pump. Use the video to check that the solvent is flowing and that the measurement table is being filled.
12. Allow the first peak in the chromatogram to build up and return back to the background count rate.
13. Select __________ solvent.
14. Allow the second peak to build up and return to the background count rate.
15. The experiment is now complete. Email the data and log book to your school email address.
16. Click on ‘logout and exit’.
Missing words/phrases:
3 M HNO3
background count rate
1ml/minute
0.0001 M HNO3
'wet' the entire column
'inject activity!'